Abstract
Introduction
Cancer associated venous thromboembolism (VTE) is associated with a significant morbidity and mortality burden in cancer care, and anticoagulation offers a survival benefit. Recurrent VTE and major bleeding complications due to secondary prophylaxis remain a salient issue in treating patients with cancer associated-VTE, regardless of validated risk assessment models. Gastrointestinal (GI) cancers confer an increased risk for recurrent VTE and bleeding. We evaluated our current institutional utilization of Direct Oral Anticoagulants (DOACs) in patients with active GI malignancies addressing both efficacy and safety profiles with these agents. We estimated the association strength between each risk factor and the outcomes to further contribute in future risk assessment tools.
Methods
This is a retrospective chart review of patients receiving DOACs with histologically proven GI malignancy and a symptomatic or incidental VTE treated at the University of Arizona Cancer Center from November 2013 to February 2017. Patients were excluded if DOACs were prescribed for any other reason not related to VTE: VTE was determined to be in the context of a non-GI malignancy or when anticoagulation was contraindicated. The primary efficacy outcome was defined as documented recurrent deep vein thrombosis (DVT), nonfatal pulmonary embolism (PE), or fatal PE before and after 6 months of DOAC therapy. The secondary safety outcome was defined as documented major bleeding (hemoglobin reduction of ≥2 g/dL, requirement of transfusion of ≥2 units of Packed Red Blood Cells, bleeding in a critical site, or bleeding contributing to death) before and after 6 months of therapy. Descriptive statistical analyses were utilized. The t- test was performed to compare continuous variables. Fisher exact test is used for testing the difference in categorical variables. Odds ratios were used to compare the relative odds of the occurrence of the outcome given exposure to the risk factor. The 95% confidence interval (CI) was used to estimate the precision of the OR. Results were determined to be 'statistically significant' when this value was less than or equal to 0.05.
Results
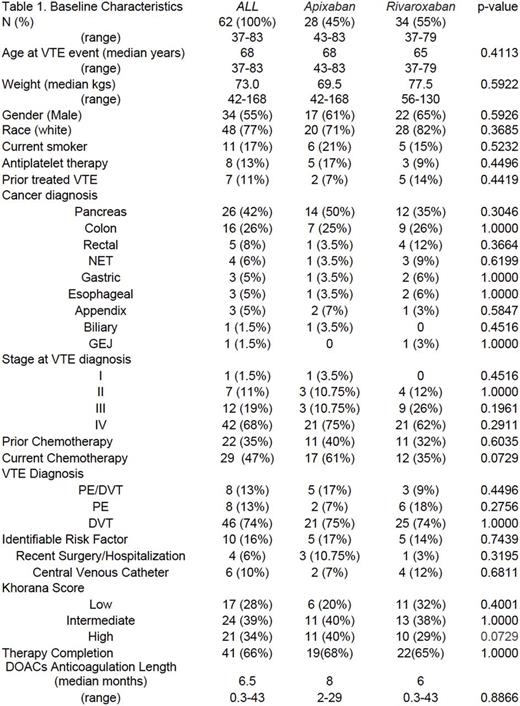
Our review included 62 patients with balanced baseline characteristics whom were prescribed apixaban (n=28) and rivaroxaban (n=34) [Table 1]. Primary outcome events at 6 months were 4.8% in total (2 DVT & 1 PE); 7.4% (2 DVT) for patients on apixaban and 2.9% (1 PE) for those taking rivaroxaban. Beyond 6 months on DOACs therapy, 6.4% of patients experienced recurrent VTE (1 DVT event in the apixiban subgroup). Secondary outcome events at 6 months were 11.2% total (7 major bleeds), 7.1% (n=2) and 14.7% (n=5) for apixaban and rivaroxaban respectively. There were 2 bleeds contributing to death (hemopericardium and upper GI bleed) and 2 other bleed in critical sites (subarachnoid hemorrhage and retroperitoneal) in the rivaroxaban group, with all other adverse outcomes being upper or lower GI bleeds. Including those events beyond 6 months, 17.7% of patients had recorded bleeding events (2 additional major bleeds for each therapy). Significant predictors of a primary or secondary outcome were pancreatic cancer (OR, 9.6, 95% CI, 1.48 to 62.16, p =0.017), stage IV disease (OR, 15, 95% CI, 7.55 to 297.63, p = 0.0075) and high Khorana Score [≥3] (OR, 7.5, 95% CI, 1.3 to 43.92, p= 0.0238). Those who suffered a primary or secondary outcome were 67 times more likely to die within a month period, compared to those who completed their therapy (CI, 5.33 to 854.82, p = 0.0011).
Conclusions
Cancer associated VTE remains a challenging clinical scenario with a lack of data for utilization of DOACs in the setting of secondary prophylaxis. The utilization of DOACs in cancer patients provides another therapy for VTE secondary prophylaxis treatment, which must be weighed, based on safety profiles in our study population. The utilization of rivaroxaban led to a higher bleeding rate in patients, with both apixaban and rivaroxaban showing similar efficacy for prophylaxis. This study warrants further exploration of DOACs in the setting of VTE treatment for patients on active chemotherapy who are at high risk of recurrent VTE. In addition, further evaluation of clinical predictors that may influence the risk of VTE recurrence and major bleeding is warranted. Wide confidence intervals reflect of our sample size and future studies may benefit from multi-center participation to maximize sample size.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal